

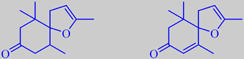
8,9-dehydro-4,5-dihydrotheaspirone and 8,9-dehydrotheaspirone
from Reseda odorata flowers
Reseda odorata (Recedaceae) Mignonette
Reseda odorata, or mignonette, is a native of Egypt and the shores of the Mediterranean. It was known to
Pliny and the Romans. In the wild it is a small plant with fragrant greenish-white flowers with red stamens, but several cultured varieties exist. Bees are fond of mignonette because of its plenty of nectar (the bee photographed is Hylaeus signatus). Mignonette has been grown in the Grasse region for perfumery purpose (by extraction).
In order to achieve highly odoriferous flowers, mignonette requires a rich, fertile, clean soil with plenty of sun and irrigation.
The flowers have an intense, sweet, violet-like and fruity odor with a green nuance.
Interestingly, the vacuum headspace concentrate of mignonette flowers contains remarkable amounts of the theaspirones shown, 4-11 % and 4-6 %, respectively. These compounds are also known as natural constituents of tobacco aroma and are, as well as the ionones, derived from carotenoid precursors. [247]
Etymology: Lat. resedare, relieving. Fr. mignonette, "little darling".




2-aminobenzaldehyde and 3(Z)-hexen-1-ol
Robinia pseudoacacia (Fabaceae) False Acacia
For unknown reasons robinia is often wrongly called acacia. Linné consequently named it pseudoacacia (Lat. pseudo, false). Acacias, however, are tropical and subtropical members of the mimosa family. Robinia is a tall, North American tree with clusters of white flowers in the springtime. Since the beginning of the 16'th century it has been planted as an ornamental tree in Europe.
The fragrance of the flowers is fine and sweet, being dominated by the aromatic aldehyde 2-aminobenzaldehyde [5]. The leaves are rich in the unsaturated aliphatic alcohol 3(Z)-hexen-1-ol, called leaf alcohol. In perfumery terms, this odorant has a typical 'green' odour reminiscent of freshly cut grass [55].
P.S. Robinia wood is resistant to rot because of its high level of flavonoids (around 6 % of the dry material), for instance robinethin, dirobinethin and taxifolin. Methanolic extracts from the wood of robinia has shown to be just as effective as traditional impregnating chemicals like pentachlorophenol and chromated copper arsenate [85].



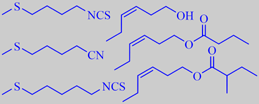
aroma impact compounds in rocket
Eruca sativa (Cruciferae) Rucola, Roquette
This crucifer, a long known salad green from the Mediterranean area, has a rich nutty-green flavour with a subdued mustard-like pungency. It is a piquant addition with lettuce, salads, as a topping on pizzas, etc.
Jirovetz et al. analysed the headspace from freshly cut rocket leaves using solid phase micro extraction with GC, GC-MS, and olfactometry. More than 50 constituents could be identified. Characteristic aroma impact compounds were found to be methylthioalkyl isothiocyanates and -nitriles together with (Z)-3-hexen-1-ol and -esters. The following compounds were found in more than 5 % concentration: 4-methylthiobutyl isothiocyanate (14 %), 5-methylthiopentanonitrile (5 %), 5-methylthiopentyl isothiocyanate (9 %), (Z)-3-hexen-1-ol (11 %), (Z)-3-hexen-1-yl butanoate (11 %), (Z)-3-hexen-1-yl 2-methylbutanoate (5 %) [178]. Likewise, Miyazawa et al. found 4-methylthiobutyl isothiocyanate and 5-methylthiopentanonitrile to be major components [179].
See also watercress.
Etymology: Lat. eruca, from Lat. roc, harsh; Lat. sativa, cultivated.


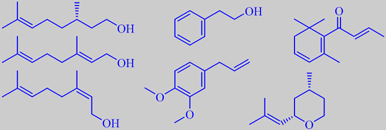
rose alcohols, methyleugenol, beta-damascenone and (-)-cis-rose oxide
Rose
Rosa damascena (Rosaceae) Damask rose
The true wild roses add up to a few hundred species from the temperate
and subtropic zones on the northern hemisphere, including North Africa,
Ethiopia, Himalaya and the Philippines. The crossings and grown varieties
are more than tenfold that number.
The rose is the most celebrated of flowers, symbol of love and beauty.
The precious rose oil is obtained by careful steam distillation of the
freshly picked flowers of the Damask rose, Rosa damascena, mainly
in Bulgaria, Turkey, Morocco and China. It takes several hundred man-hours
to pick the 3-4 tonnes of flowers needed to produce 1 kg of rose oil,
which makes rose oil one of the most expensive essential oils.
Following the development of organic chemistry, continued investigations
have been carried out to identify the
odour impact compounds in rose oil. Prior to 1959 no real progress was
achieved in spite of the identification of all constituents present in
more than 1 % concentration (corresponding to 83 % of the oil). A break-through
came in 1959 with the discovery of (-)-cis-rose oxide, present in 0.5
% concentration. This cyclic monoterpene ether is responsible for the
highly volatile, floral-green top note in rose oil. Moreover, in 1970,
beta-damascenone was identified in 0.1 % concentration, supplying a floral-fruity
character with a plum-like shading. Because of the extremely low odour
threshold of this compound, it has a much higher odour impact than citronellol,
present in 330 times greater concentration [3]. However, rose oxide and
beta-damascenone appear to be rearrangement products that are formed during
the steam distillation process from labile progenitors [87]. The major
constituents of rose oil are (-)-citronellol, certain specific paraffines,
geraniol and nerol, phenethyl alcohol, and methyleugenol - in decreasing
order. Some of the important minor- and trace constituents are (-)-cis-rose
oxide, beta-damascenone, beta-ionone, beta-damascone, 1-p-menthen-9-al,
and rose furan. More than 350 compounds have been identified [5] [88]
[89].

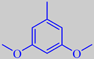
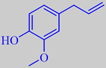
3,5-dimethoxytoluene
The name 'tea rose' is the result of a misunderstanding - the rose was created in the Fa Tee nursery near Canton
in China! In 1810 it was discovered by an Englishman who sent a specimen to England. It is the ancestor behind several yellow and yellowish modern roses.
However, the scent of these roses is rather different from that of traditional roses. Surprisingly, their scent is strongly influenced by the aromatic compound orcinyl dimethyl ether, or 3,5-dimethoxy-toluene, which is chemically related to the oakmoss odorants [89]. This phenol ether has a warm and sweet, nut-like, earthy-mossy odour - according to Arctander.




eugenol
The rugosa rose origins in Japan or northern China (it is also called Kamchatka rose). It is a hardy bush with densely thorny stems and plenty of root suckers. The leaves have a corrugated surface and are shiny green. Rugosa rose has become an invasive specimen in several parts of the world. In Denmark, for example, it spreads along the coastline as it thrives in sandy soils, outperforming native plant communities (the seeds float and tolerate salty waters for several months). Nevertheless, the fragrant flowers and fleshy hips are beloved.
The flowers have most of their volatiles in common with the damask rose, but are characterized by a spicy note from eugenol in their deep sweetness [130]. An interesting, complete phytochemical review of Rosa rugosa was undertaken by Hashidoko [131].
The palette of rosy-smelling odorants is large. One newer achievement utilizes the
fact that the isobutenyl group common of many terpenes is rather isosteric with a phenyl group. By "replacing" the isobutenyl group with a phenyl group in citronellol, for example, the odour character isn't changed much, but instead the more substantive odorant Phenoxanol ® (IFF) is obtained. The vapor pressure of Phenoxanol is ten times lower than that of citronellol. However, the odour threshold is also about ten times lower! Moreover, Phenoxanol has a richer and smoother odour than citronellol [43]. Similarly nerol oxide, another trace constituent of rose oil, has been mimicked by Givaudan's Rosyrane Super, an extremely diffusive odorant with green, geranium-like and metallic notes and closely resembling the odor of pelargonium leaves.
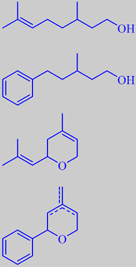
citronellol and Phenoxanol ®,
nerol oxide and Rosyrane Super

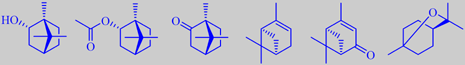
(+)-borneol, (+)-bornyl acetate, (+)-camphor, (+)-alpha-pinene, (+)-verbenone and 1,8-cineole
Rosemary
Rosmarinus officinalis (Labiatae)
Rosemary is a typical member of the Mediterranean coastal 'macchia' vegetation.
It is an aromatic shrub with an intense and pleasant smell. In the antiquity
rosemary was associated with Aphrodite, the goddess of love. Although
the only member of the genus Rosmarinus, it is found in numerous
chemotypes and cultivars.
Rosemary oil is obtained by steam distillation of the flowering tops,
mainly in Spain, Morocco and Tunisia. One of the first perfumes made by
distillation was based on rosemary oil, namely the Hungarian queen's-water,
'Aqua Reginae Hungariae'. It is told that the Hungarian Queen Isabella,
suffering heavily from rheumatism, used this perfume with beneficial results.
She became young and beautiful again, and the King of Poland offered to
marry her.
Good qualities of rosemary oil have a pleasant fresh odour with characteristic
resinous nuances and slightly bitter undertones. (+)-Borneol and (+)-verbenone
are important constituents. (+)-Borneol has a dry-camphoraceous, woody-peppery
odour, (+)-verbenone possesses an odour reminiscent of camphor, menthol
and celery. (+)-Alpha-pinene, (+)-bornyl acetate, (+)-camphor and 1,8-cineole
are characteristic too [90] [91] [92].
Rosemary oil is extensively used in perfumery. The dried and broken leaves
are used as a spice.
Etymology: Lat. rosmarinus, from Lat. ros, dew, and
Lat. marinus, sea.



(-)-linalool and (+)-linalool
Aniba rosaeodora (Lauraceae), Brazilian rosewood
Brazilian rosewood oil, or Bois de rose oil, once a valuable essential oil in perfumery, is steam-distilled from the chipped wood of Aniba rosaeodora from Brazil, Peru and Guyana. It has a balsamic, floral, slightly rose-like odor.
It was formerly an important source of linalool but is no longer competitive as such. In the sixties synthetic linalool became available at a much lower price, and due to raiforest eradication the species is now threatened by habitat loss.
Anyway, rosewood oil has more to offer than pure linalool. Its more spicy and complex piquancy can, for example, transform a lily-of-the-valley type perfume and bring it to life, whereas synthetic linalool cannot, having a flatter and more one-dimensional effect [226].
The linalool percentage in oil from various parts of A. rosaeodora may be as high as 73-99 %. In trunk wood the
(-)-linalool stereoisomer is dominating (close to 100 %) whereas (+)-linalool becomes more abundant in thin parts and branches and reaches 85 % in the leaves [227].
Etymology: linalool, from linaloe oil (from Bursera sp.) in which it was originally identified.
P.S.
Less specifically, rosewood refers to a number of richly hued tropical timbers of reddish brown color with darker veining, some of them, for example, from trees of the Fabacean family.



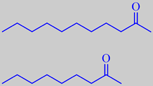
2-undecanone, 'rue ketone',
and 2-nonanone
Ruta graveolens (Rutaceae) Common rue
Rue is chiefly mentioned here because it has given name to the rue family, Rutaceae, including many fragrant plants, not least the Citrus species. There are about 60 species of the genus Ruta having a natural distribution from the Canary Islands via the Mediterranean countries and Central Europe to eastern Siberia. Common rue is a perennial herb from the Mediterranean area. It has been grown as a medicinal plant, for instance in Switzerland, Austria and southern Germany, where it may be found growing wild, especially on warm and dry, calcareous ground. It was always kept in the gardens of the monasteries as it was considered to be of benefit to chastity.
The whole plant has a penetrating odour due to an essential oil with a great prevalence of straight chain aliphatic
2-ketones. 2-Undecanone or 'rue ketone' (47 %), and 2-nonanone (19 %) are the major constituents [93].